Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
 7 surface using high-resolution core-level photoelectron spectroscopy
7 surface using high-resolution core-level photoelectron spectroscopyKakiuchi, Takuhiro*; Matoba, Tomoki*; Koyama, Daisuke*; Yamamoto, Yuki*; Kato, Daiki*; Yoshigoe, Akitaka
Surface Science, 701, p.121691_1 - 121691_8, 2020/11
Times Cited Count:1 Percentile:6.04(Chemistry, Physical)Ultrathin hafnium films on Si(111)-7 7 were studied using synchrotron radiation photoelectron spectroscopies to reveal the chemical states at interface and surface. Ultrathin Hf layers grow on clean Si(111)-7
7 were studied using synchrotron radiation photoelectron spectroscopies to reveal the chemical states at interface and surface. Ultrathin Hf layers grow on clean Si(111)-7 7 surface by lever rule. Surface and interface of Hf/Si(111) contain three components (metallic Hf layers, Hf monosilicide (HfSi) and Si-rich Hf silicide). Ultrathin Hf layers changes HfSi
7 surface by lever rule. Surface and interface of Hf/Si(111) contain three components (metallic Hf layers, Hf monosilicide (HfSi) and Si-rich Hf silicide). Ultrathin Hf layers changes HfSi islands on bared Si(111)-7
islands on bared Si(111)-7 7 surface after annealing at 1073 K. It was found that the long axes of the rectangle islands expand the direction connecting the corner holes in DAS model of clean Si(111)-7
7 surface after annealing at 1073 K. It was found that the long axes of the rectangle islands expand the direction connecting the corner holes in DAS model of clean Si(111)-7 7 surface.
7 surface.
Takakuwa, Yuji*; Ishizuka, Shinji*; Yoshigoe, Akitaka; Teraoka, Yuden; Moritani, Kosuke; Ogawa, Shuichi*; Mizuno, Yoshiyuki*; Tonda, Hideki*; Homma, Teiichi*
Shinku, 47(6), p.457 - 461, 2004/06
The oxidation reaction of Ti(0001) surface by oxygen molecules was observed by real-time in-situ photoemission spectroscopy using synchrotron radiation in the conditions of 400 C and 3.7
C and 3.7 10
10 Pa. The uptake curve of oxygen showed a plateau at the dose of 45-85 L and then increased again. This re-growth of oxide film was attributed to the drastic change of Ti oxidation state from TiO to TiO
Pa. The uptake curve of oxygen showed a plateau at the dose of 45-85 L and then increased again. This re-growth of oxide film was attributed to the drastic change of Ti oxidation state from TiO to TiO . Consequently, the characteristic change of the uptake curve was caused by the oxidation state change of Ti atoms.
. Consequently, the characteristic change of the uptake curve was caused by the oxidation state change of Ti atoms.
 1 surface during oxidation controlled by translational kinetic energy of O
1 surface during oxidation controlled by translational kinetic energy of O at room tempearture
at room tempeartureYoshigoe, Akitaka; Teraoka, Yuden
Surface Science, 532-535, p.690 - 697, 2003/06
Times Cited Count:22 Percentile:69.44(Chemistry, Physical)no abstracts in English
Yoshigoe, Akitaka; Moritani, Kosuke; Teraoka, Yuden
Shinku, 46(5), p.424 - 428, 2003/05
In order to study the reaction mechanism of the initial thermal Si(001) oxidation by O (1
(1 10
10 Pa) at the surface temperature region between 870K and 1120K, we have performed the
Pa) at the surface temperature region between 870K and 1120K, we have performed the 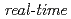 synchrotron radiation photoemission spectroscopy of Si-2p and O-1s levels. The oxygen uptake curves as a function of the oxygen exposure time were analyzed on the basis of kinetics. The Si oxidation states corresponding to the adsorbed oxygen amount were well clarified by the time evolution of Si-2p photoemission spectra.
synchrotron radiation photoemission spectroscopy of Si-2p and O-1s levels. The oxygen uptake curves as a function of the oxygen exposure time were analyzed on the basis of kinetics. The Si oxidation states corresponding to the adsorbed oxygen amount were well clarified by the time evolution of Si-2p photoemission spectra.
Omichi, Toshihiko*; Arai, Yasuo
Journal of Nuclear Science and Technology, 39(Suppl.3), p.156 - 159, 2002/11
no abstracts in English
 analysis using synchrotron radiation photoemission spectroscopy for initial oxidation of oxygen preadsorbed Si(001) surfaces induced by supersonic O
analysis using synchrotron radiation photoemission spectroscopy for initial oxidation of oxygen preadsorbed Si(001) surfaces induced by supersonic O molecular beams
molecular beamsYoshigoe, Akitaka; Teraoka, Yuden
Surface and Interface Analysis, 34(1), p.432 - 436, 2002/08
Times Cited Count:3 Percentile:8.65(Chemistry, Physical)no abstracts in English
 using synchrotron radiation photoemission spectroscopy
using synchrotron radiation photoemission spectroscopyYoshigoe, Akitaka; Teraoka, Yuden
Shinku, 45(3), p.204 - 207, 2002/03
no abstracts in English
Muramatsu, Yasuji; Kuramoto, Kentaro*; Gullikson, E. M.*; Perera, R. C. C.*
Surface Review and Letters, 9(1), p.267 - 270, 2002/02
Times Cited Count:5 Percentile:30.61(Chemistry, Physical)no abstracts in English

Yoshigoe, Akitaka; Teraoka, Yuden
Shinku, 44(3), p.195 - 198, 2001/03
no abstracts in English
J. A. BERRY*; M. BROWNSWORD*; D. J. ILETT*; Linklater, C. M.*; Mason, C.*; TWEED, C. J.*
JNC TJ8400 2000-060, 60 Pages, 2000/02
Batch sorption experiments have been carried out to investigate the sorption behaviour of plutonium onto basalt and sandstone from the appropriate rock-equilibrated waters under different redox eonditions. Redox Potentials in solution were controlled by the addition of two reducing agents and one oxidising agent. Thermodynamic chemical modelling was undertaken to interpret the results. The sorption models were based on iron oxide. They adequately reproduced the data for sorption of plutonium onto sandstone, but tended to underpredict sorption onto basalt.
G M N BASTON*; J A BERRY*; M BROWNSWORD*; D J LLETT*; C M LINKLATER*; S W SWANTON*; Tweed, C. J.*
JNC TJ8400 99-078, 72 Pages, 1999/03
A desk study has been carried out to establish the feasibility of measuring the oxidation state of plutonium under near-neutral strongly-reducing conditions. X-ray absorbance spectroscopy appears to be capable of establishing the oxidation state of plutonium sorbed on a suitable substrate. An experimental and modelling investigation has been performed to study the sorption of plutonium onto basalt, mudstone and sandstone under strongly-reducing conditions at three concentrations of carbonate. Appropriate synthetic rock-equilibrated de-ionised water and seawater were used. A model has been developed to describe the sorption of plutonium onto basalt, mudstone and sandstone in de-ionised water and seawater. Predicted R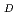 values are generally in good agreement with the observed experimental measurements. The model is based on sorption of plutonium(III) species and assumes iron oxide is the dominant sorbing phase.
values are generally in good agreement with the observed experimental measurements. The model is based on sorption of plutonium(III) species and assumes iron oxide is the dominant sorbing phase.
 M
M CuO
CuO (M=alkali metals), synthesized in MOH molten salts
(M=alkali metals), synthesized in MOH molten saltsSasaki, Yuji; Wuryanto*; Takeishi, Hideyo
Analytical Sciences, 12, p.499 - 501, 1996/06
Times Cited Count:1 Percentile:5.9(Chemistry, Analytical)no abstracts in English
Yoshida, Zenko; Aoyagi, Hisao; ; Takeishi, Hideyo; Sasaki, Yuji; Uno, Seiichiro; Tachikawa, Enzo
Journal of Alloys and Compounds, 213-214, p.453 - 455, 1994/00
Times Cited Count:8 Percentile:54.8(Chemistry, Physical)no abstracts in English
 or Cu
or Cu in a superconducting disk sample by flow-coulometry
in a superconducting disk sample by flow-coulometrySasaki, Yuji
Superconductors, Surfaces and Superlattices (Trans. of Materials Research Soc. Jpn., Vol. 19A), 0, p.301 - 304, 1994/00
no abstracts in English
 in high-T
in high-T superconducting La
superconducting La Sr
Sr CuO
CuO pellet by flow-coulometry
pellet by flow-coulometrySasaki, Yuji; Aoyagi, Hisao; Takeishi, Hideyo; Yoshida, Zenko
Physica C, 191, p.347 - 353, 1992/00
Times Cited Count:7 Percentile:55.21no abstracts in English
Ashida, Takashi; Sonobe, Hitoshi; Yamada, Kazuo
PNC TN8600 91-003, 38 Pages, 1991/06
no abstracts in English
Kimura, Takaumi;
Reza Kenkyu, 18(4), p.279 - 288, 1990/04
no abstracts in English
G.T.Seaborg*; Yamashita, Toshiyuki
Nihon Genshiryoku Gakkai-Shi, 31(7), p.805 - 811, 1989/07
Times Cited Count:0 Percentile:0.46(Nuclear Science & Technology)no abstracts in English
*;
Water Res., 23(1), p.43 - 50, 1989/00
Times Cited Count:16 Percentile:67.71(Engineering, Environmental)no abstracts in English
; ; *
Anal. Chem., 59, p.400 - 405, 1987/00
Times Cited Count:35 Percentile:84.9(Chemistry, Analytical)no abstracts in English